VITA™ - the New Point-of-Care CD4 Counter
Improved functionality and data quality
Higher throughput
Reduced cartridge cost
State of art technology
Analytical Performance of the VITA™ Point-of-care CD4 Enumeration Assay
https://www.medrxiv.org/content/10.64898/2025.12.16.25342340v1.full.pdf
Chui Mei Ong1,2 , Aquiles R. Henriquez-Trujillo3 , Melissa Alamillo1,2, Wei Huang4 , Ed Goldberg4 , Dorien Van den Bossche3 , Tinne Gils3* & Alan H.B. Wu1,2*
1. University of California, San Francisco, United States 2. San Francisco General Hospital, San Francisco, United States 3. Institute of Tropical Medicine, Department of Clinical Sciences, Antwerp, Belgium 4. Accesso Biotech Corporation, Santa Clara, United States
VITA Point of Care CD4 Test Demo Video
Accesso VITA™ POC CD4 Test Demo Video
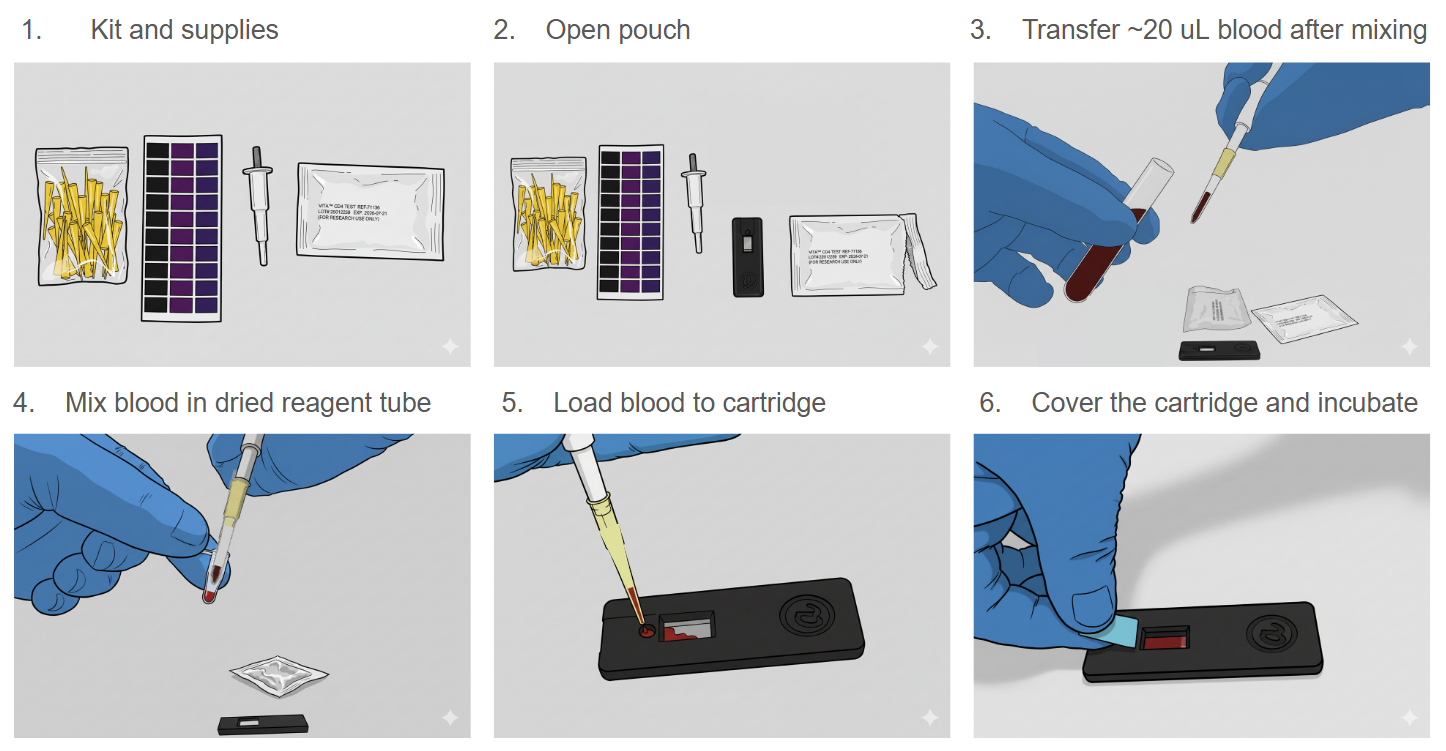
Simply mix approximately 20 uL whole blood sample in the dried reagent tube and add to the cartridge for 30 min incubation at room temperature; Measurement on the instrument takes about 1-2 min to get the results. No accurate sample volume pipetting and counting bead required.
“Access to point-of-care tests for people with advanced HIV under threat as diagnostics corporations to stop production” — Medecins Sans Frontieres (Doctors Without Borders) May 11, 2023
“With the crisis worsening, many HIV programmes in LMICs either have no access to CD4 testing or have access to only one novel, lateral-flow-based CD4 semi-quantitative assay that increasingly shows its limitations in terms of diagnostic performance and feasibility, particularly when used at the point of care as opposed to in controlled laboratory settings” - The Lancet - Global Health, 2025
For More Information About the VITA™ POC CD4 Test
Email: info@accessobio.com
Phone/text: +1 408-780-8187
Regulatory status may vary by region. Please consult your local distributor for specific details